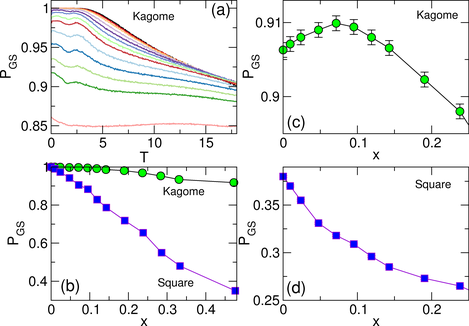
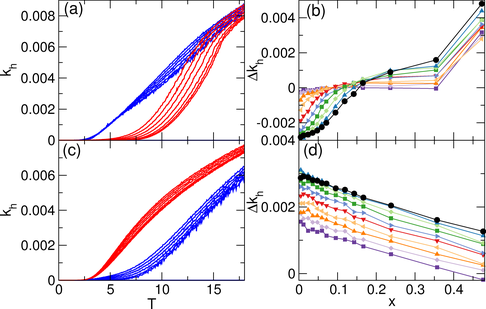
 Figure 5: (a) Hopping rate kh vs T for the square ice system.
Blue curves: khc for vertices adjacent to doubly-occupied traps;
red curves: khf for vertices not adjacent to doubly-occupied traps.
The curves are plotted for
x = 0, 0.00945, 0.0236, 0.0472, 0.0709, 0.0945,
and 0.1181, from the bottom red (blue) curve to the top red (blue) curve.
The hopping rate is enhanced near the doping sites in the square ice.
(b) The difference in the hopping rates
∆kh=khf−khc
vs x for
T=8 (black circles),
T=9 (dark blue up triangles),
T=10 (light green diamonds),
T=11 (dark green squares),
T=12 (light blue right triangles),
T=13 (red down triangles),
T=14 (light orange left triangles),
T=15 (dark orange up triangles),
T=16 (light purple diamonds),
and T=17 (dark purple squares).
For small values of x,
∆kh is negative since the hopping rate is
highest close to the doping sites, and it approaches zero as the doping level
or temperature is increased.
(c)
khc (blue) and khf (red) vs T for the kagome ice
at x = 0, 0.0094, 0.0238, 0.0476, 0.07142, 0.0952, and 0.119
from the bottom red (blue) curve to the top red (blue) curve.
Here the trend in the hopping rate is reversed,
with the lowest hopping rates close to the doping sites.
(d) δkh vs x for the kagome ice for T=8 to 17 from top to
bottom, with the same symbols as in panel (b).
Figure 5: (a) Hopping rate kh vs T for the square ice system.
Blue curves: khc for vertices adjacent to doubly-occupied traps;
red curves: khf for vertices not adjacent to doubly-occupied traps.
The curves are plotted for
x = 0, 0.00945, 0.0236, 0.0472, 0.0709, 0.0945,
and 0.1181, from the bottom red (blue) curve to the top red (blue) curve.
The hopping rate is enhanced near the doping sites in the square ice.
(b) The difference in the hopping rates
∆kh=khf−khc
vs x for
T=8 (black circles),
T=9 (dark blue up triangles),
T=10 (light green diamonds),
T=11 (dark green squares),
T=12 (light blue right triangles),
T=13 (red down triangles),
T=14 (light orange left triangles),
T=15 (dark orange up triangles),
T=16 (light purple diamonds),
and T=17 (dark purple squares).
For small values of x,
∆kh is negative since the hopping rate is
highest close to the doping sites, and it approaches zero as the doping level
or temperature is increased.
(c)
khc (blue) and khf (red) vs T for the kagome ice
at x = 0, 0.0094, 0.0238, 0.0476, 0.07142, 0.0952, and 0.119
from the bottom red (blue) curve to the top red (blue) curve.
Here the trend in the hopping rate is reversed,
with the lowest hopping rates close to the doping sites.
(d) δkh vs x for the kagome ice for T=8 to 17 from top to
bottom, with the same symbols as in panel (b).
|