Bicontinuous Networks as Complex Systems
with Adaptive Functionality
Professor Alan Heeger
University of California at Santa Barbara
Department of Physics
4415 Broida Hall
Santa Barbara, CA 93106
(805) 893-3184
(805) 893-4755 (FAX)
Because there is no entropy of mixing for blends of high molecular weight polymers, phase separation is expected on very general grounds (the entropy of mixing goes to zero as 1/N, where N is the polymerization index). Functionalized surfactants can be used to control the phase separated morphology.
A sketch of a material with fibrillar, bicontinuous network morphology is shown below. Imagine, for example, that the dark component is a metallic polymer (such as polyaniline. PANI) and the white component is the insulating host polymer (e.g. PMMA). Because of the self-assembled network morphology, electrical conductivity is achieved at remarkably low volume fractions of the PANI-CSA and without degradation of the attractive mechanical properties of the host engineering polymer; the blends are also optically transparent since the mesh size can be well below optical wavelengths.

There is a need for a deeper understanding of the chemical and physical principles that underly such self-assembly, particularly the formation of bicontinuous network morphologies. There is an opportunity to exploit these principles to make new heterogeneous structures with potential applications in electronic, optical, or biomedical devices, catalysis, and separations.
The use of functionalized surfactants to induce network formation was introduced with initial emphasis on creating conducting polymer blends with ultra-low percolation thresholds. Because of the bicontinuous networks, there are connected (conducting) pathways so that the blend is rendered conducting at low volume fractions. In particular, PANI can be doped and, simultaneously, rendered soluble in the conducting form in organic solvents by reacting with functionalized protonic acids of the form H+(M--R), in which the counterion species, (M--R), consists of a polar M- = SO3- covalently bonded to the R functional group[1]. The bifunctional counter-ion acts like a "surfactant" in that the M- is ionically bound to the charged, protonated chain (to form a salt), and the R-group is chosen to be compatible with nonpolar or weakly polar organic liquids (for solubility) or the host polymer (for blending). An illustrative example is dodecyl benzene sulfonic acid (DBSA), where RDBSA= -f-C12H25 and f is a phenyl ring. The surfactant counterion-induced processibility enabled the fabrication of conducting polyblends with traditional host polymers. After doping and complexing with DBSA (or camphor sulfonic acid, CSA), the conducting PANI complex can be co-dissolved with a host polymer in an organic solvent at a desired ratio to form a solution of the polyblend. The polyblend can then be processed from solution to yield conducting, films, fibers, etc.
The PANI blends are remarkable in that electrical conductivities of 1 S/cm are obtained in polyblends containing only 2% of the conductive component with the percolation threshold occurring at volume fractions as low as fc Ľ 0.3%. The onset of electrical conductivity at such low volume fractions results from the network morphology, with connected pathways even at remarkably low volume fractions of the conducting PANI-complex. The fibrillar network was imaged by transmisson electon microscopy; TEM micrographs of PANI-CSA in PMMA show the PANI-CSA network clearly and confirm that the network becomes disconnected at volume fractions between 0.25% and 0.5%. At concentrations near fc, the structure of the interconnected fibrillar network is self-similar, consistent with the well-known result that all systems are fractal near the percolation threshold on length scales below the correlation length of the percolating cluster. The fractal structure of the network was confirmed through numerical analysis of the TEM micrographs.
The network of conducting pathways that self-assemble in blends of PANI with insulating host polymers results from a compromise: the counter-ions want to be at the interface between the polar PANI (a salt) and the weakly polar host, whereas the PANI and host tend to phase separate. The result is a phase separated structure with high surface area, like that shown in the introductory sketch. Although the existence of the network morphology can be rationalized in these terms, a deeper understanding of the issues involved in the self-assembly of networks is lacking. Electron micrographs indicate that when the surface to volume ratio of the PANI-CSA segregated regions becomes too large, the connected network structure cannot be maintained. The micrographs demonstrate a minimum diameter of order a few tens of nm for the fibrillar links; this minimum dimension is not understood.
The use of 'surfactant' counterions was introduced with the goal of making polyaniline processible in the conducting form. The self-assembly of phase separated networks was an unexpected --- but very welcome --- bonus. The generalization to a larger class of conducting polyblends with network morphology is already well underway. A few examples follow:
(i) žBulk HeterojunctionÓ Materials from Bicontinuous Networks
In this case, the dark component of the introductory sketch would be a semiconducting polymer as donor [a soluble semiconducting polymer] and the other component would be an acceptor [e.g. fullerene derivatives or electronegative polymers]. Ultrafast photoinduced electron transfer has been demonstrated between these components with quantum efficiency approaching unity! The phase separated network morphology enabled the creation of žbulk heterojunction materialsÓ; i.e. network materials in which the entire volume is within a few nm of a heterojunction. Using bulk heterojunction materials for photoinduced charge separation and charge collection, high efficiency žplasticÓ solar cells have been demonstrated; the carrier collection efficiency (~29 % electrons/photon) and energy conversion efficiency (~2.9 %) of these D/A network polymer photovoltaic cells are the best obtained from organic materials, better by more than two orders of magnitude than devices made without the use of bifunctional network materials. These devices are excellent photodetectors; better than commercial UV enhanced silicon for wavelengths less than 600 nm, and suitable for use as the active detector elements in digital cameras.
The efficient charge separation results from photoinduced electron transfer; the high collection efficiency results from a bicontinuous network of internal donor/acceptor heterojunctions. Control of the phase separated network through surfactant addition and through design and synthesis of suitable acceptors are the route to further improvements.
(ii) Bifunctional Network Blends for Polymer Light-Emitting Electrochemical Cells
The LEC is a new device for light-emission from electroactive polymers. In the LEC, a p-n junction is created in-situ through simultaneous p-type and n-type electrochemical doping on opposite sides of a thin film of conjugated polymer that contains added electrolyte to provide the necessary counterions for doping. Polymer LECs with blue, green, and orange emission have been fabricated that turn on at voltages less than 3V. The active medium of an LEC is a semiconducting, luminescent polymer blended with a solid electrolyte [e.g. poly(ethylene oxide), PEO, containing (+) and (-) ions] to provide the counterions needed for p- and n-type doping. This blend is sandwiched between metal electrodes.
The key to high performance LECs is, once again, the phase separated network; the microstructure of the semiconducting (luminescent) polymer/polymer electrolyte blend which yields the best LEC performance achieved to date is shown in the figure below.
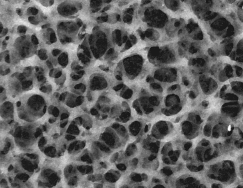
TEM micrograph of MEH-PPV + PEO/Li + bifunctional additive (octylcyanoacetate) in the ratio 1:1:1 spin cast from cyclohexanone showing the bicontinuous network morphology (1 cm = 80 nm). The phase separated network morphology is clearly seen.
In this case, the light component is the semiconducting and luminescent polymer [such as MEH-PPV, a soluble derivative of poly(phenylene vinylene)] and the dark component is the polymer electrolyte [PEO containing a suitable concentration of lithium triflate]. The blend was coaxed into the network structure by using a bifunctional (žsurfactant-likeÓ) additive, octylcyanoacetate, polar on one end and nonpolar on the opposite end. The bifunctional network morphology minimizes the distance required for ionic diffusion from the electrolyte into the polymer during electrochemical doping. The result is faster response (faster turn-on), improved brightness and improved efficiency.
There is much to be learned here, a whole new class of materials to be explored, and much to be gained from improved LEC performance. For example, although the reason for the faster response is clear, the origins of the improved efficiency and brightness are not clear. More importantly, we must gain a deeper understanding of the self-assembly of such network microstructured blends rather than proceed by trial and error.
In summary, Bicontinuous Networks as Complex Systems with Adaptive Functionality offer a number of interesting and potentially important opportunities for electro-active polymers, especially in the area of "plastic electronic" devices. The need to utilize two (or perhaps more) incompatible components to achieve the desired material properties leads naturally to phase separated structures. High performance requires bicontinuous networks and a deep understanding of how to create and control the microstructure of such networks in heterogeneous polymer composite materials.