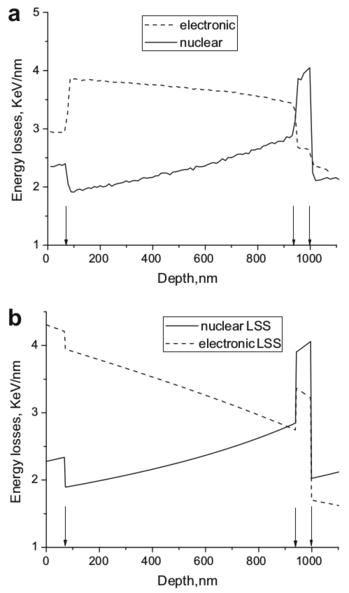
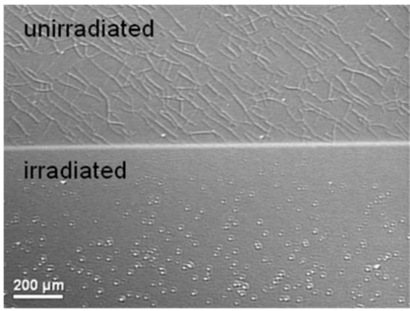
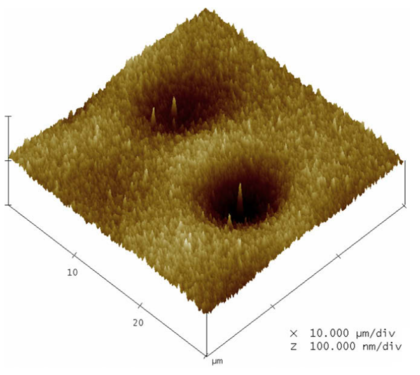
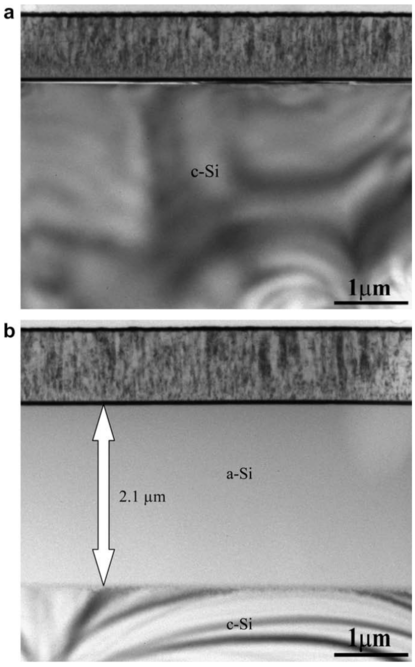
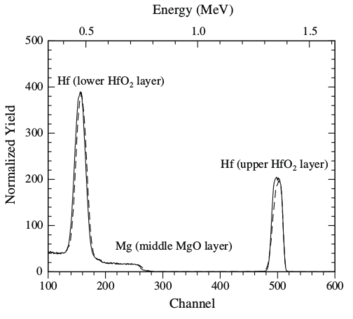
| Layer | Layer areal density, RBS (at./cm2) | Layer thickness, TEM (nm) | Calculated density, ρ (at./cm3) | Calculated porosity, × 100 (%) |
| Upper HfO2 | 4.3 × 1017 | 71 | 6.0 × 1022 | 27 |
| MgO | 8.7 × 1018 | 869 | 1.0 × 1023 | 6 |
| Lower HfO2 | 4.0 × 1017 | 59 | 6.7 × 1022 | 18 |
Table 1:
Areal densities and thicknesses of individual layers in the unirradiated
HfO2/MgO/HfO2 tri-layer sample,
as determined by RBS and TEM, respectively. Layer densities were
estimated using
ρ = nRBS/tTEM,
where areal densities, nRBS,
and thicknesses tTEM, are in units of at/cm2
and cm, respectively. Porosity was estimated as
(1 - ρ /ρth), where ρ and ρth
are the calculated and theoretical densities, respectively.
Theoretical densities, ρth,
for HfO2 and MgO were assumed to be
8.22 × 1022 and 1.07 × 1023 at./cm3, respectively.
|